Efficacy of atropine eyedrops in reducing myopia progression and axial elongation in myopic children: a meta-analysis
Abstract
Purpose: To determine the efficacy of various concentrations of atropine eyedrops on retarding myopia progression and axial elongation in Asian children.
Study design: Meta-analysis.
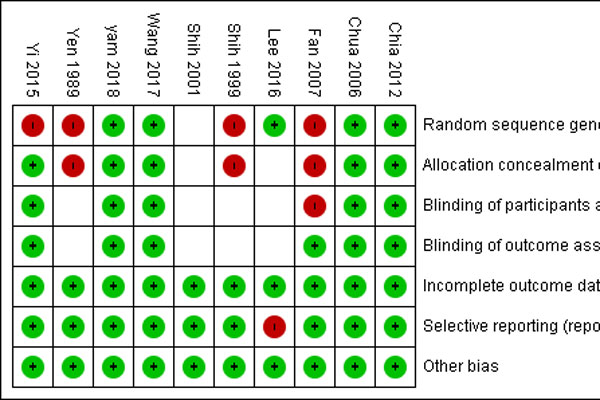
Methods: Randomized clinical trials and prospective interventional non-randomized studies which enrolled children aged 4 to 14 years old who received atropine treatment for myopia were included in the study. The Cochrane Collaboration 6 aspects of bias was used to assess the risk of bias for all included studies. Outcome measures were myopia progression and axial elongation. Meta-analysis was conducted using the random-effects model.
Results: Eight randomized clinical trials and two prospective interventional non-randomized studies which included a total of 1,229 Asian children were included in the analysis. The pooled mean difference between control and atropine for myopia progression was 0.77 diopters (D) per year [CI 0.64, 0.89]. Subgroup analysis by concentration showed a decreasing trend with decreasing concentration. The pooled mean difference of myopia progression for 1%, 0.5%, 0.25%, and 0.1–0.125% atropine was 0.97 D/year [CI 0.72, 1.21], 0.88 D/year [CI 0.74, 1.02], 0.79 D/year [CI 0.37, 1.21], and 0.80 D/year [CI 0.62, 0.97], respectively; whereas that for 0.01% atropine was 0.46 D/year [CI -0.02, 0.94] indicating that this intervention may or may not be favorable for slowing myopia progression. The pooled mean difference between control and atropine for axial elongation was -0.22 mm [CI -0.29, -0.14] favoring atropine. Subgroup analysis by concentration also showed decreasing trend with decreasing concentration. The pooled mean difference of axial elongation for 1%, 0.5%, 0.1%, 0.05%, and 0.025% atropine was -0.44 mm [CI -0.57, -0.32], -0.19 mm [CI -0.35, -0.04], -0.10 mm [CI -0.17, -0.03], -0.21 mm [CI -0.28, -0.14], and -0.12 mm [CI -0.16, -0.08], respectively; whereas that for 0.01% atropine was -0.01 mm [CI -0.09, 0.06] indicating that this intervention may or may not be favorable in reducing axial elongation.
Conclusion: This meta-analysis shows that the effects of atropine for both myopia progression and axial elongation are dose-dependent for the concentration 0.025% to 1%. Results for 0.01% atropine are still equivocal.
References
Gong Q, Janowski M, Luo M, Wei H, Chen B, Yang G, Liu L. Efficacy and Adverse Effects of Atropine in Childhood Myopia: A Meta-analysis. JAMA Ophthalmol. 2017;135(6):624-630. https://doi.org/10.1001/jamaophthalmol.2017.1091.
Pineles SL, Kraker RT, VanderVeen DK, et al. Atropine for the Prevention of Myopia Progression in Children. Ophthalmology. 2017;124(12):1857-1866. https://doi.org/10.1016/j.ophtha.2017.05.032. Epub 2017 Jun 29. PMID: 28669492.
Song YY, Wang H, Wang BS, Qi H, Rong ZX, Chen HZ. Atropine in Ameliorating the Progression of Myopia in Children with Mild to Moderate Myopia: A Meta-analysis of Controlled Clinical Trials. J Ocul Pharmacol Ther. 2011;27(4):361-8. https://doi.org/10.1089/jop.2011.0017. Epub 2011 Jun 7.
Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology. 2019;126(1):113-124. https://doi.org/10.1016/j.ophtha.2018.05.029.
Li SM, Wu SS, Kang MT, et al. Atropine Slows Myopia Progression More in Asian than White Children by Meta-analysis. Optom Vis Sci. 2014;91(3):342-350. https://doi.org/10.1097/OPX.0000000000000178.
Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2011;(12):CD004916. https://doi.org/10.1002/14651858.CD004916.pub3
Chia A, Chua WH, Cheung YB, et al. Atropine for the Treatment of Childhood Myopia: Safety and Efficacy of 0.5%, 0.1%, and 0.01% Doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-354. https://doi.org/10.1016/j.ophtha.2011.07.031. Epub 2011 Oct 2.
Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, Hung PT. Effects of Different Concentrations of Atropine on Controlling Myopia in Myopic Children. J Ocul Pharmacol Ther. 1999;15(1):85-90.
Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL. An Intervention Trial on Efficacy of Atropine and Multi-focal Glasses in Controlling Myopic Progression. Acta Ophthalmol. 2001;79(3):233-236.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928
Lee CY, Sun CC, Lin YF, Lin KK. Effects of topical atropine on intraocular pressure and myopia progression: a prospective comparative study. BMC Ophthalmol. 2016;16:114. https://doi.org/10.1186/s12886-016-0297-y
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: www.handbook.cochrane.org [accessed November 21, 2018].
Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol. 1989;21(5):180-182, 187.
Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285-2291. Epub 2006 Sep 25. PMID: 16996612
Fan DS, Lam DS, Chan CK, Fan AH, Cheung EY, Rao SK. Topical atropine in retarding myopic progression and axial length growth in children with moderate to severe myopia: a pilot study. Jpn J Ophthalmol. 2007;51(1):27-33. Epub 2007 Feb 9.
Yi S, Huang Y, Yu SZ, Chen XJ, Yi H, Zeng XL. Therapeutic effect of atropine 1% in children with low myopia. J AAPOS. 2015;19(5):426-429. https://doi.org/10.1016/j.jaapos.2015.04.006. Epub 2015 Jul 27. PMID: 26228967
Wang YR, Bian HL, Wang Q. Atropine 0.5% eyedrops for the treatment of children with low myopia: A randomized controlled trial. Medicine (Baltimore). 2017 Jul;96(27):e7371. https://doi.org/10.1097/MD.0000000000007371.
Huang J, Wen D, Wang Q, et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-analysis. Ophthalmology. 2016;123(4):697-708. https://doi.org/10.1016/j.ophtha.2015.11.010
Copyright (c) 2021 Stacey Anne Sy Sau, Alvina Pauline Dy Santiago, Maria Dulce Taino Peralta, Aliana Jimenez Vera Cruz, Jimmy Jarvis Chua Lo

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work twelve (12) months after publication simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).


