Safety and efficacy of reconstituted atropine 0.01% eye drops for Indian children with myopic progression
Abstract
Aim: To evaluate safety and efficacy of reconstituted atropine 0.01% eye drops for Indian children with myopic progression.
Methods: Fifty children with progressive myopia with their spherical equivalent increasing at least 0.75 D in 6 months (0.75 to 1.50 D) were put on reconstituted atropine 0.01%. Ocular examination, including near vision, near point of accommodation (NPA), pupil size and axial length, was done. Subjective symptoms of glare and photophobia were noted. Systemic side effects were documented. Analysis was done using Microsoft Excel 2010.
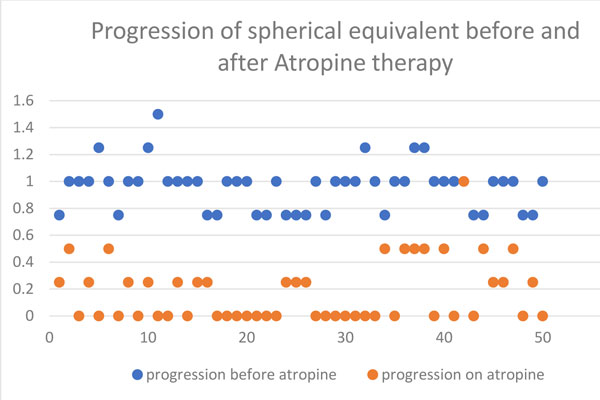
Results: The average age of patients was 9.5 years (range 5 to 14 years) and they were followed up for 1 year. Average mesopic and photopic pupil size was 5 and 4 mm, respectively. Average NPA was 9 cm. Mean increase in spherical equivalence was 0.18 D over 6 months. Average increase in axial length was 0.12 ± 0.11 mm over 6 months and 0.2 ± 0.29 mm over 1 year. Average increase in spherical equivalent over 6 months was 0.07 ± 0.19 D and over 1 year was 0.32 ± 0.29 D. No systemic side effects were recorded.
Conclusion: Reconstituted atropine 0.01% eye drops is safe and efficacious in slowing
the progression of myopia in Indian children.
References
World Health Organization. Elimination of Avoidable Visual Disability due to Refractive Errors (WHO/PBL/0079). Geneva, Switzerland: World Health Organization; 2000:Vision 2020.
Saw S-M. A synopsis of the prevalence rates and environmental risk factors for myopia. Clin Exp Optom. 2003;86:289-294.
Jain IS, Jain S, Mohan K. The epidemiology of high myopia—changing trends. Indian J Ophthalmol. 1983;31:723-728.
Mohan M, Pakrasi S, Zutshi R. Myopia in India. Acta Ophthalmol Suppl. 1988;185:19-23.
Goss DA, Hampton MJ, Wickham MG. Selected review on genetic factors in myopia. J Am Optom Assoc. 1988;59:875-884.
Mutti DO. Myopia—the nature versus nurture debate goes on. Invest Ophthalmol Vis Sci. 1996;37(6):952-957.
Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol 1989;21:180-182, 187.
Shih YF, Chen CH, Chou AC, et al. Effects of different concentrations of atropine on controlling myopia in myopic children. J Ocul Pharmacol Ther. 1999;15:85-90.
Shih YF, Hsiao CK, Chen CJ, et al. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand. 2001;79:233-236.
Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285-2291.
Luu CD, Lau AM, Koh AH, Chua WH, Balakrishnan V, Tan D. Effects of long term atropine usage on retinal function. Invest Ophthalmol Vis Sci. 2003;44:4790.
Chia A, Chua W-H, Cheung Y-B, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology. 2012;119(2):347-354.
Wu PC, Yang YH, Fang PC. The long-term results of using low concentration atropine eye drops for controlling myopia progression in schoolchildren. J Ocul Pharmacol Ther. 2011;27(5):461-466.
Loughman J, Flitcroft DI. The acceptability and visual impact of 0.01% atropine in a Caucasian population. Br J Ophthalmol. 2016;100(11):1525-1529.
Nishiyama Y, Moriyama M, Fukamachi M, et al. Side effects of low dose atropine. Nippon Ganka Gakkai Zasshi. 2015;119(11):812-816.
Copyright (c) 2020 Vidhya C., Kaushik Murali, Sowmya R.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work twelve (12) months after publication simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).


