Amiodarone-induced vortex keratopathy at a low maintenance dose
Abstract
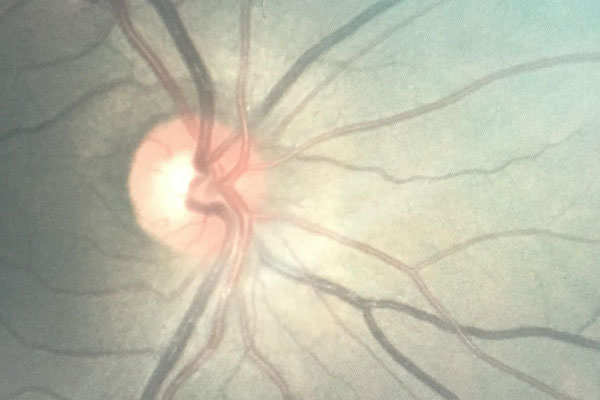
Vortex keratopathy is a common side effect of amiodarone, which is a class III antiarrhythmic agent. We describe a 50-year-old man who developed vortex keratopahy with amiodarone 200 mg BD for atrial fibrillation since two years. The daily (400 mg/day) and cumulative dose (100 g) combined with the length of therapy is associated with the toxicity. Toxic effects may also be observed at lower maintenance doses, as observed in this patient. This case indicates that multi-organ toxicity due to amiodarone may develop even with short-term use and a low maintenance dose. Having been off the medication for two months, it is expected that the deposition pattern will diminish, as is the case for the vast majority of patients.
References
Gittinger JW, Jr, Asdourian GK. Papillopathy caused by amiodarone. Arch Ophthalmol. 1987;105:349-351.
Hollander DA, Aldave AJ. Drug-induced corneal complications. Curr Opin Ophthalmol. 2004;15:541-548.
D’Amico DJ, Kenyon KR, Ruskin JN. Amiodarone keratopathy: drug-induced lipid storage disease. Arch Ophthalmol. 1981;99:257-261.
Flach AJ, Dolan BJ, Sudduth B, Weddell J. Amiodarone-induced lens opacities. Arch Ophthalmol. 1983;101:1554-1556.
Uçakhan OO, Kanpolat A, Ylmaz N, Ozkan M. Amiodarone keratopathy: an in vivo confocal microscopy study. Eye Contact Lens. 2005;31:148-157.
Copyright (c) 2020 Deepti Mahajan

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work twelve (12) months after publication simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).


